Introduction
One of the commonest genetic abnormalities seen in adult Acute Lymphoblastic Leukemia (ALL) is the reciprocal translocation between chromosomes (Chr.) 9 and 22, leading to the formation of the BCR-ABL fusion gene. This manifests as a shortened Chr 22 (Philadelphia chromosome, Ph+ALL). Despite the widespread use of tyrosine kinase inhibitors (TKI) which target the BCR-ABL fusion protein, Ph+ALL is considered high risk with more relapses and lesser long-term cures. The Haematology Cancer Consortium (HCC) maintains a multi-center database of several hematological malignancies. The ALL database was established in 2018, and the data of the Ph+ALL patients entered into the registry are presented here. The baseline presenting features and induction outcomes were available for all patients, while the survival data is yet to mature.
Methods
Prospective data of patients (≥ 18 years) with Ph+ ALL (Diagnosed between January 2018 to December 2022) from 10 centers were entered into an independent centralized online data capture system and analyzed for presenting characteristics, treatment, and survival outcomes. Ph+ ALL was defined by the demonstration of t(9,22) [conventional karyotype/ Fluorescent in situ hybridization (FISH)] or the demonstration of BCR-ABL (by polymerase chain reaction) at diagnosis. Treatment (protocol for ALL, TKI used) was per individual-center preference. Intensive protocols (MCP-841, BFM-95, COG), predominantly meant for children, were labeled as “pediatric-type,” and less intense protocols (GMALL, Hyper CVAD, UKALL) were considered “adult-type”.
Results
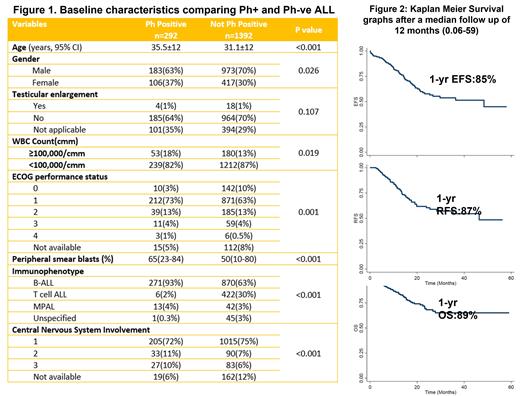
In the four years, 1684 patients with ALL registered and 1409 had been tested for Phor BCR-ABL gene, and 292 were labeled as Ph+ALL [Males: 183(63%), Median age: 35 years (18-77); Subtype: B -ALL: 271(93%), T-ALL: 6 (2%), Mixed phenotype: 13 (4%) ; CNS 2 or 3: 60 (18%)]. Of these, 289 underwent treatment. “Pediatric-type” protocols (BFM, MCP841, ICICLE) were used in 241 (84%) patients, followed by the GMALL protocol in 44 patients (15%) and others in 5 patients. TKIs were used in 234 patients (81%) [Imatinib (29%) and Dasatinib (49%)]. Figure 1 shows the baseline characteristics. Induction complications (all grades) were as follows: tumor lysis (18%), pneumonia (11%), neuropathy (15%), pancreatitis (4%), venous thrombosis (5%), and hyperglycemia (44%).
After induction, 72% achieved complete remission (CR), 8% were refractory, and 8% died during induction. The rest (12%) were lost from follow-up during induction and were not evaluable. Post-induction minimal residual disease (MRD) status was assessed in 177 patients and was positive in 75 (42%). To date, 21 patients have undergone allogenic transplants.
Survival analysis: After a median follow-up of 12 months (0.06-59), 61 patients have relapsed and 57 have died, and 52 (18%) have been lost from follow-up. The estimated 1-year event-free (EFS), relapse-free (RFS), and overall survival (OS) were 85% (S.E:+/-0.027), 87% (+/- 0.033), and 89%(+/- 0.024) respectively ( Figure 2). No factors affecting prognosis were identified in the current analysis.
Conclusions
In a large prospective database of 12 centers, we found that most adult Ph+ALLs in India are treated with a pediatric-type intensive protocol. Dasatinib is the preferred TKI, followed by imatinib. There was a high degree of treatment abandonment, with an 18% loss from follow-up within six months (including 12% during the induction phase).
Disclosures
Jain:Intas Pharmaceuticals: Research Funding; Zydus Pharmaceuticals: Research Funding; ImmunoACT: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal